ICAO Standard Atmosphere - ISA¶
| Author: | D.Thaler |
|---|---|
| Created: | Aug 2013 |
| Last changed: | 2019-08-11 |
Definitions and Equations
Definitions¶
The ICAO standard atmosphere (ISA) is defined within the meteorological scope as a reference atmosphere for mainly avionic purposes. The atmosphere is considered to be a dry ideal gas. The values of physical constants are shown in the following table:
| Constants for the ICAO standard atmosphere | |
|---|---|
| constant gravity | \(g_0=\) 9.80665 m/s² |
| constant earth radius | \(R=\) 6 356 766 m |
| molar mass of dry air | \(M_d=\) 28.9644 kg/kmol |
| universal gas constant | \(R_*=\) 8 314.32 J/(kmol K) |
| gas constant for dry air | \(R_d=R_*/M_d \approx\) 287.053 J/(kg K) |
ISA starts with surface values of temperature and pressure and continues with different levels of thermal stratification. Levels above 32 km are defined in ISA in 1993 but are neglected here.
| h [km] | T [C] | \(\gamma\) [K/m] | p [hPa] |
|---|---|---|---|
| 0 | +15.0 | 0.0065 | 1013.25 |
| 11 | −56.5 | 0.0000 | |
| 20 | −56.5 | -0.0010 | |
| 32 | −44.5 |
Equations¶
Within this framework all necessary equations can be derived. Starting point is the hydrostatic approximation of the vertical component of the equation of motion, the so called hydrostatic equation:
that states an equilibrium between vertical pressure gradient force per unit mass and gravity.
Inserting the ideal gas equation of dry air
yields after some simple transformations
Integration between the limits \(h_0\) and \(h\) and exponentiation of the result gives the generalized barometric equation
with the pressure values \(p = p(h)\) and \(p_0 = p(h_0)\).
Isothermal Atmosphere¶
Given a vertically constant temperature \(T(z) = T_0\) the above equation simply gives
The inverse function gives the geopotential height as function of the pressure difference for an isothermal atmosphere (a.k.a. hypsometric equation):
that can be used for altimetry.
With the ideal gas equation a very similar expression can be derived for the density of air
Vice versa the density altitude can be calculated as:
In ISA the isothermal equations are true for the “tropopause”-level between 11 km and 20 km altitude.
Politropic Atmosphere¶
From the surface up to 11 km and from 20 km to 32 km the temperature in ISA is not constant but shows a linear change with height:
( \(\lambda = \gamma / T_0\) ).
In equation (3) the integral must be solved between \(z = h_0\) and \(z = h\):
That finally gives for pressure as function of geopotential height:
Solving the above equation for \(h\) gives the hypsometric equation for the polytropic atmosphere:
Density as a function of height can easily be derived by the ideal gas equation \(\rho(h) = p(h)/(R_d T(h))\):
Inverting the equation gives a relation between height and density (density altitude) for the polytropic atmosphere:
with \(\beta = \left[g_0/(R_d\gamma) -1\right]^{-1}\)
Results¶
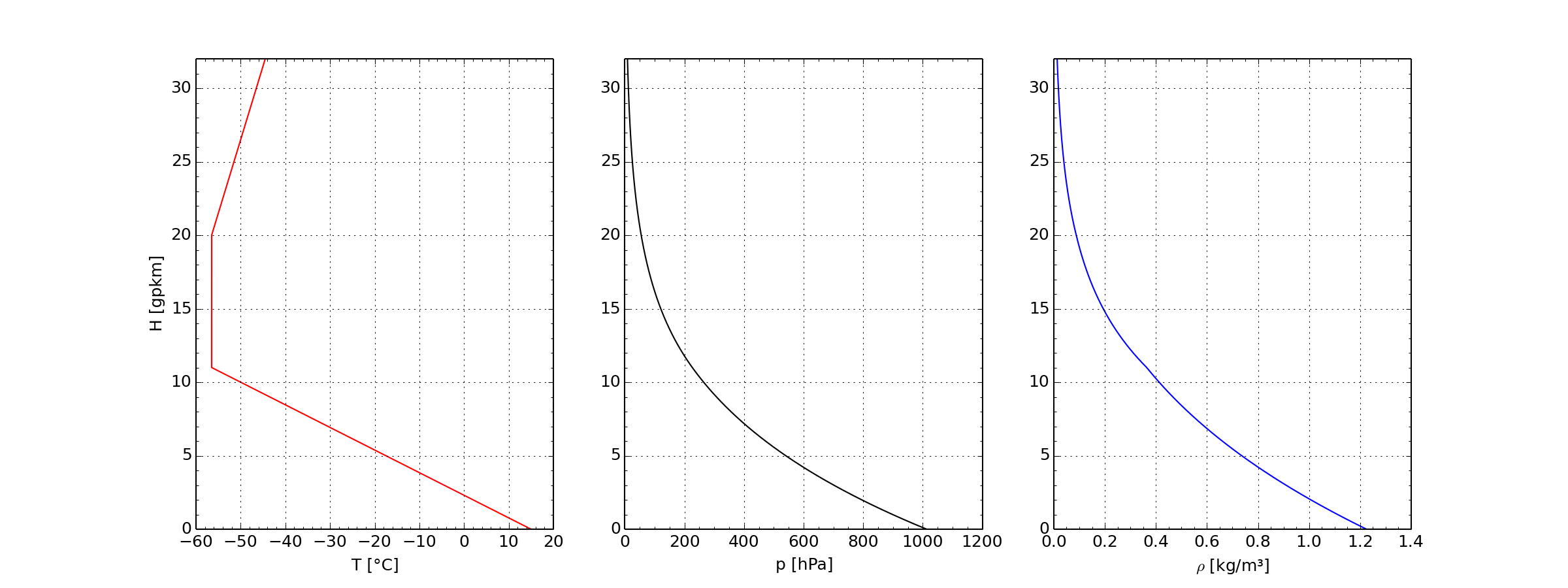
ICAO standard atmosphere up to 32 km height
- Left: Temperature in Celsius, Center: Pressure in Hektopascal,
- Right: Density in Kilogram per Cubic Meter
The graphic was produced with the help of the Python program
isaplots.py which also serves
as demo program for the use of the Python library adisalib (for
download in https://python3.foehnwall.at/adisalib.html or
https://python2.foehnwall.at/adisalib.html ).
A table with an appropriate selection of values can be seen here:
| h[gpm] | T[C] | p[hPa] | \(\rho\) [kg/m³] |
|---|---|---|---|
| -300 | 16.95 | 1049.81 | 1.2608 |
| 0 | 15.00 | 1013.25 | 1.2251 |
| 500 | 11.75 | 954.60 | 1.1673 |
| 1000 | 8.50 | 898.74 | 1.1117 |
| 1500 | 5.25 | 845.55 | 1.0581 |
| 2000 | 2.00 | 794.94 | 1.0065 |
| 2500 | -1.25 | 746.81 | 0.9569 |
| 3000 | -4.50 | 701.07 | 0.9092 |
| 4000 | -11.00 | 616.38 | 0.8192 |
| 5000 | -17.50 | 540.18 | 0.7361 |
| 6000 | -24.00 | 471.79 | 0.6597 |
| 7000 | -30.50 | 410.58 | 0.5895 |
| 8000 | -37.00 | 355.97 | 0.5252 |
| 9000 | -43.50 | 307.40 | 0.4663 |
| 10000 | -50.00 | 264.34 | 0.4127 |
| 11000 | -56.50 | 226.30 | 0.3639 |
| 12000 | -56.50 | 193.28 | 0.3108 |
| 14000 | -56.50 | 141.00 | 0.2267 |
| 16000 | -56.50 | 102.86 | 0.1654 |
| 18000 | -56.50 | 75.03 | 0.1207 |
| 20000 | -56.50 | 54.74 | 0.0880 |
| 24000 | -52.50 | 29.30 | 0.0463 |
| 26000 | -50.50 | 21.53 | 0.0337 |
| 28000 | -48.50 | 15.86 | 0.0246 |
| 30000 | -46.50 | 11.72 | 0.0180 |
| 32000 | -44.50 | 8.68 | 0.0132 |
Table of symbols¶
| \(h\) | geopotential height [gpm] |
| \(p\) | pressure [Pa] |
| \(T\) | Temperature [K] |
| \(z\) | geopotential height [gpm] |
| \(\gamma\) | vertical temperature gradient \((-\partial T/\partial z)\) [K/m] |
| \(\rho\) | air density [kg/m³] |
References¶
- Standard meteorological textbook, e.g. Michael Hantel: Einführung Theoretische Metetrologie, Springer-Spektrum, Springer Verlag Berlin-Heidelberg 2013
- ICAO Standard Atmosphäre (Wikipedia-de)
- ICAO Standard Atmosphere (Wikipedia-en)
Any comments to info at foehnwall dot at